The boron–boron triple bond? A thermodynamic and force field based interpretation of the N-heterocyclic carbene (NHC) stabilization procedure†
Abstract
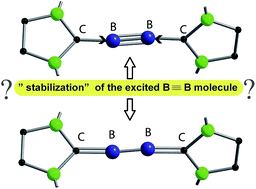
Recently, the NHC→B![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) B←NHC molecule 1 has been published in Science where it is described as a stabilized B2 molecule in its 1Σ excited state (B2*) (NHC = N-heterocyclic carbene C3N2H2(C6H3Pri2-2,6)2). The bonding of 1 based on sophisticated calculations and the BB distances of the solid compound was discussed as the first example of a B2 triple bond in a stable molecule. Now we present an only experimentally based interpretation of 1via detailed thermodynamic considerations, including its fragmentation to B2 molecules. Furthermore, from the vibrational spectrum force constants (fBB for the BB bond and fBC for the BC bond) were extracted, which are classical examples to indicate single, double and triple CC bonds in organic chemistry. The consequence of both properties of 1 (ΔE and f) generates a new interpretation which is in contrast to the triple bond donor–acceptor description visualized by arrows and which casts a critical light on the interpretation of any NHC “stabilized” molecule.
B←NHC molecule 1 has been published in Science where it is described as a stabilized B2 molecule in its 1Σ excited state (B2*) (NHC = N-heterocyclic carbene C3N2H2(C6H3Pri2-2,6)2). The bonding of 1 based on sophisticated calculations and the BB distances of the solid compound was discussed as the first example of a B2 triple bond in a stable molecule. Now we present an only experimentally based interpretation of 1via detailed thermodynamic considerations, including its fragmentation to B2 molecules. Furthermore, from the vibrational spectrum force constants (fBB for the BB bond and fBC for the BC bond) were extracted, which are classical examples to indicate single, double and triple CC bonds in organic chemistry. The consequence of both properties of 1 (ΔE and f) generates a new interpretation which is in contrast to the triple bond donor–acceptor description visualized by arrows and which casts a critical light on the interpretation of any NHC “stabilized” molecule.

 Please wait while we load your content...
Please wait while we load your content...