Entropy driven chain effects on ligation chemistry†
Abstract
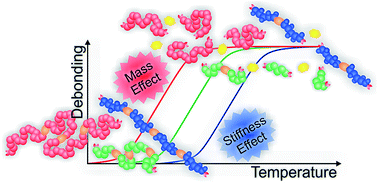
We report the investigation of fundamental entropic chain effects that enable the tuning of modular ligation chemistry – for example dynamic Diels–Alder (DA) reactions in materials applications – not only classically via the chemistry of the applied reaction sites, but also via the physical and steric properties of the molecules that are being joined. Having a substantial impact on the reaction equilibrium of the reversible ligation chemistry, these effects are important when transferring reactions from small molecule studies to larger or other entropically very dissimilar systems. The effects on the DA equilibrium and thus the temperature dependent degree of debonding (%debond) of different cyclopentadienyl (di-)functional poly(meth-)acrylate backbones (poly(methyl methacrylate), poly(iso-butyl methacrylate), poly(tert-butyl methacrylate), poly(iso-butyl acrylate), poly(n-butyl acrylate), poly(tert-butyl acrylate), poly(methyl acrylate) and poly(isobornyl acrylate)), linked via a difunctional cyanodithioester (CDTE) were examined via high temperature (HT) NMR spectroscopy as well as temperature dependent (TD) SEC measurements. A significant impact of not only chain mass and length with a difference in the degree of debonding of up to 30% for different lengths of macromonomers of the same polymer type but – remarkably – as well the chain stiffness with a difference in bonding degrees of nearly 20% for isomeric poly(butyl acrylates) is found. The results were predicted, reproduced and interpreted via quantum chemical calculations, leading to a better understanding of the underlying entropic principles.

 Please wait while we load your content...
Please wait while we load your content...