A counteranion triggered arylation strategy using diaryliodonium fluorides†
Abstract
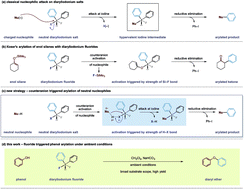
A mild and transition metal-free counteranion triggered arylation strategy has been developed using diaryliodonium fluorides. The fluoride counteranion within the hypervalent iodonium species displays unusual reactivity that activates a phenolic O–H bond leading to electrophilic O-arylation. A wide range of phenols and diaryliodonium salts are compatible with this transformation under remarkably mild conditions. Furthermore, we pre-empt the wider implications of this strategy by demonstrating the compatibility of the arylation tactic with latent carbon nucleophiles.

 Please wait while we load your content...
Please wait while we load your content...