Stabilization of H3+ in the high pressure crystalline structure of HnCl (n = 2–7)
Abstract
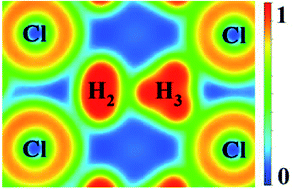
The particle-swarm optimization method has been used to predict the stable high pressure structures up to 300 GPa of hydrogen-rich group 17 chlorine (HnCl, n = 2–7) compounds. In comparison to the group 1 and 2 hydrides, the structural modification associated with increasing pressure and hydrogen concentration is much less dramatic. The polymeric HCl chains already present in the low temperature phase under ambient pressure persist in all the high pressure structures. No transfer of electrons from the chlorine atoms into the interstitial sites is found. This indicates the chemical bonding at high pressure in group 17 elements is fundamentally different from the alkali and alkaline elements. It is found that almost perfectly triangular H3+ ions can be stabilized in the crystalline structure of H5Cl.

 Please wait while we load your content...
Please wait while we load your content...