Abstract
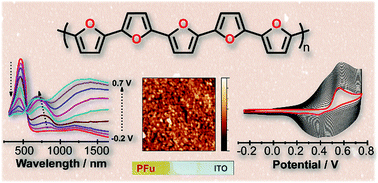
Polyfurans have never been established as useful conjugated polymers, as previously they were considered to be inherently unstable and poorly conductive. Here, we show the preparation of stable and conducting polyfuran films by electropolymerization of a series of oligofurans of different chain lengths substituted with alkyl groups. The polyfuran films show good conductivity in the order of 1 S cm−1, good environmental and electrochemical stabilities, very smooth morphologies (roughness 1–5 nm), long effective conjugation lengths, well-defined spectroelectrochemistry and electro-optical switching (in the Vis-NIR region), and have optical band-gaps in the range of 2.2–2.3 eV. A low oxidation potential needed for polymerization of oligofurans (compared to furan) is a key factor in achievement of improved properties of polyfurans reported in this work. DFT calculations and experiments show that polyfurans are much more rigid than polythiophenes, and alkyl substitution does not disturb backbone planarity and conjugation. The obtained properties of polyfuran films are similar or superior to the properties of electrochemically prepared poly(oligothiophene)s under similar conditions.


 Please wait while we load your content...
Please wait while we load your content...