Ligand design for Rh(iii)-catalyzed C–H activation: an unsymmetrical cyclopentadienyl group enables a regioselective synthesis of dihydroisoquinolones†
Abstract
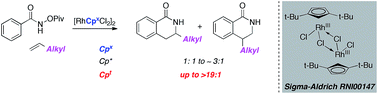
We report the regioselective synthesis of dihydroisoquinolones from aliphatic alkenes and O-pivaloyl benzhydroxamic acids mediated by a Rh(III) precatalyst bearing sterically bulky substituents. While the prototypical Cp* ligand provides product with low selectivity, sterically bulky Cpt affords product with excellent regioselectivity for a range of benzhydroxamic acids and alkenes. Crystallographic evidence offers insight as to the source of the increased regioselectivity.

 Please wait while we load your content...
Please wait while we load your content...