Structure–activity relationship studies of cyclopropenimines as enantioselective Brønsted base catalysts†
Abstract
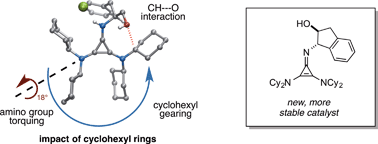
We recently demonstrated that chiral cyclopropenimines are viable Brønsted base catalysts in enantioselective Michael and Mannich reactions. Herein, we describe a series of structure–activity relationship studies that provide an enhanced understanding of the effectiveness of certain cyclopropenimines as enantioselective Brønsted base catalysts. These studies underscore the crucial importance of dicyclohexylamino substituents in mediating both reaction rate and enantioselectivity. In addition, an unusual catalyst CH⋯O interaction, which provides both ground state and transition state organization, is discussed. Cyclopropenimine stability studies have led to the identification of new catalysts with greatly improved stability. Finally, additional demonstrations of substrate scope and current limitations are provided herein.

 Please wait while we load your content...
Please wait while we load your content...