Mechanistic investigations reveal that dibromobimane extrudes sulfur from biological sulfhydryl sources other than hydrogen sulfide†
Abstract
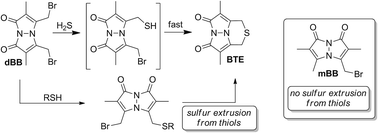
Hydrogen sulfide (H2S) has emerged as an important biological signaling molecule in the last decade. During the growth of this field, significant controversy has arisen centered on the physiological concentrations of H2S. Recently, a monobromobimane (mBB) method has been developed for the quantification of different biologically-relevant sulfide pools. Based on the prevalence of the mBB method for sulfide quantification, we expand on this method to report the use of dibromobimane (dBB) for sulfide quantification. Reaction of H2S with dBB results in formation of highly-fluorescent bimane thioether (BTE), which is readily quantifiable by HPLC. Additionally, the reaction of sulfide with dBB to form BTE is significantly faster than the reaction of sulfide with mBB to form sulfide dibimane. Using the dBB method, BTE levels as low as 0.6 pM can be detected. Upon use of the dBB method in wild-type and CSE−/− mice, however, dBB reports significantly higher sulfide levels than those measured using mBB. Further investigation revealed that dBB is able to extract sulfur from other sulfhydryl sources including thiols. Based on mechanistic studies, we demonstrate that dBB extracts sulfur from thiols with α- or β-hydrogens, thus leading to higher BTE formation than from sulfide alone. Taken together, the dBB method is a highly sensitive method for H2S but is not compatible for use in studies in which other thiols are present.

 Please wait while we load your content...
Please wait while we load your content...