Site and stereoselectivity in sulfa-Michael addition to equivocally activated conjugated dienes†
Abstract
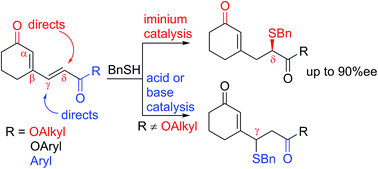
The regiochemical course of sulfa-Michael addition of thiols to terminally divergently activated dienes partially incorporated in a cyclic system, as in substituted 2-(3-oxocyclohexenyl)-vinyls, can be to some extent altered by the choice of activation method. The ratio of products of competing 1,4- and 1,6-addition reactions is dependent on the potency of the electron withdrawing group. The iminium ion catalysis favors 1,6-addition to the cyclic ketone group, changing the product preference for dienes terminated with ketone and aryl ester groups. Application of 9-epi-aminoquinine analogues and an acid allowed for both regioselective and enantioselective (up to 90% ee) addition at the distant δ position.

 Please wait while we load your content...
Please wait while we load your content...