An efficient and metal free synthesis of benzylpyridines using HI through the deoxygenation reaction†
Abstract
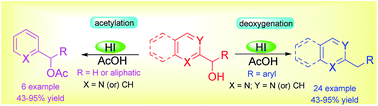
An efficient and practical method for the synthesis of benzylpyridine derivatives has been developed using aqueous hydroiodic acid in acetic acid. This method is also successfully applied for the synthesis of 2,6-disubstituted pyridine derivatives under the same reaction conditions. Using readily available aqueous hydroiodic acid as a reducing agent made this process economical. When the aryl group of the secondary alcohol is replaced by an alkyl group, the reaction gives exclusively an acetoxylation instead of deoxygenation reaction.

 Please wait while we load your content...
Please wait while we load your content...