A family of unsymmetrical hydroxyl-substituted BEDT-TTF donors: syntheses, structures and preliminary thin film studies†
Abstract
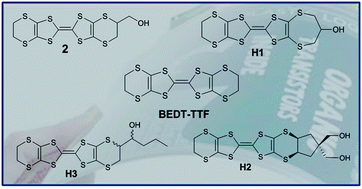
Three new unsymmetrical hydroxyl-functionalized donors H1–H3 closely related to hydroxymethyl-BEDT-TTF have been synthesised and characterised. Cyclic voltammetry studies showed that the compounds exhibit reversible two one-electron redox processes typical for BEDT-TTF derivatives. X-ray diffraction studies of H1 and H2 reveal π-stacking interactions between pairs of donors that are organized into distinct H-bonded square motifs and DFT calculations indicate that the HOMO is located on the central 1,3-dithiole rings. Protection of the hydroxyl group with acetyl in 13 eliminates co-facial S⋯S interactions between the dimers to accommodate the bulkier side chains, but short edge-to-edge S⋯S contacts offer an alternative pathway for electron mobility. Chemical oxidation of H1 and HMET 2 with I2 afforded single crystals of two 1 : 1 charge transfer salts, 18 and 19. The molecules pack as dimers with close π-stacking interactions between pairs of radical cations whose crystal structures are further stabilized via an interplay of S⋯S and S⋯I contacts. Iodine-doped surface conducting polystyrene blend films of H3 deposited on a silica substrate exhibit quasiconducting properties, but afford no OFET response when fabricated into devices. Visible-NIR studies of a doped polystyrene blend film of H3 cast on a glass substrate show absorption bands at λ = 950 and 3000 nm, consistent with mixed valence states due to the presence of charge-transfer species on the surface of the films.

 Please wait while we load your content...
Please wait while we load your content...