Catalyst free production of partial glycerides: acetone as solvent†
Abstract
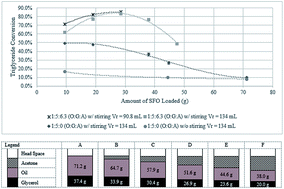
Glycerolysis of sunflower oil was studied using acetone as the solvent. Reactions were carried out at 200 to 250 °C without the need of conventional catalyst. The use of acetone in glycerolysis of oil allowed the reaction to be carried out at 250 °C and 1.8 MPa, resulting in a product that comprises 4.5% solketal, 5.8% FFA, 49.2% MG, 33.5% DG and 7% TG in 2 h. The important parameters investigated in this study were acetone addition, stirring and reactor loading. This new approach in the synthesis of partial glycerides (monoglycerides and diglycerides) avoids the use of chemical catalyst, thus avoiding unnecessary wastewater production. The partial glycerides produced may be utilized in may areas of food and pharmaceutical industries. Moreover the use of acetone as solvent allows the process to be carried out at lower operating pressures compared to other non-catalytic glycerolysis and at the same time co-produces solketal, having a wide application in fuels, pharmaceuticals and chemical synthesis.

 Please wait while we load your content...
Please wait while we load your content...