Copper-catalyzed trifluoromethylation of organic zinc reagents with an electrophilic trifluoromethylating reagent†
Abstract
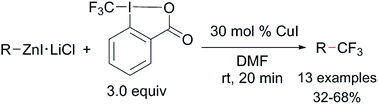
A copper-catalyzed trifluoromethylation of aryl, vinyl and alkyl zinc reagents with Togni's reagent was described. Mechanistic studies indicated that the aryl group is initially transferred from the zinc reagent to hypervalent iodine to form a tri-substituted hypervalent iodine intermediate. Consequent reductive-elimination via a concerted bond-forming step and/or radical pathway from this intermediate generates the trifluoromethylated arenes.

 Please wait while we load your content...
Please wait while we load your content...