Near threshold photodissociation study of EMIMBF4 vapor
Abstract
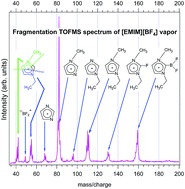
Photodissociation of the [EMIM][BF4] ionic liquid vapors following excitation with light in the vacuum ultraviolet region was studied at different liquid temperatures. For this purpose time-of-flight mass spectra and partial ion yield spectra were recorded. From the partial ion yield measurements the appearance energies of the main positive fragments were determined. The appearance energies of the two dominating fragments were determined to be 8.2 eV (MIM+) and 7.4 eV (dehydrogenated EMIM+). The second threshold energy of the dehydrogenated EMIM cation was found to be 9.4 eV. The supporting ab initio calculations explain and agree with these experimentally determined energy values. EMIMF and EMIMBF2 cations were also identified as possible photodissociation fragments. The possible presence of hydrogenated and dehydrogenated EMIM cations was shown. The [EMIM][BF4] ion-pair was shown to dissociate after photoexcitation (even below the ionization threshold). No significant thermal decomposition of the [EMIM][BF4] molecules was observed.

 Please wait while we load your content...
Please wait while we load your content...