One pot synthesis of acridine analogues from 1,2-diols as key reagents†
Abstract
Lead tetraacetate have been demonstrated to be efficient, low cost and mild reagents for the one pot synthesis of acridine derivatives from a variety of 1,2-diols. 1,2-Diols are oxidised in situ to aldehydes, which in turn undergo reaction with dimedone and ammonium acetate to yield acridine derivatives. The attractive features of this process are mild reaction conditions, short reaction times, broad functional group tolerance, easy isolation of products and excellent yields. Thus, the current method utilises 1,2-diols instead of benzaldehydes to synthesise acridines derivatives.
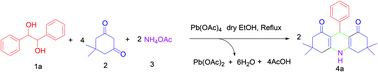

 Please wait while we load your content...
Please wait while we load your content...