Mechanistic investigations of a bifunctional squaramide organocatalyst in asymmetric Michael reaction and observation of stereoselective retro-Michael reaction†
Abstract
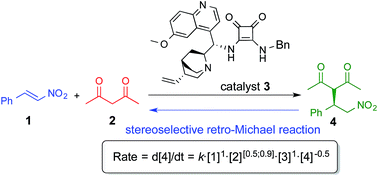
The mechanism of the addition of acetylacetone to β-nitrostyrene catalyzed by a cinchona based squaramide catalyst was studied in detail under synthetically relevant conditions. The reaction was monitored by in situ IR and 1H-NMR spectroscopy and a reaction mechanism was proposed based on these kinetics experiments. It was found that the reaction shows nearly first order dependence on both substrates and catalyst. Our investigations also revealed that the catalyst was able to promote stereoselective retro-Michael reaction.

 Please wait while we load your content...
Please wait while we load your content...