Expedient, catalyst-free, three-component synthesis of fused tetrahydropyridines in water†
Abstract
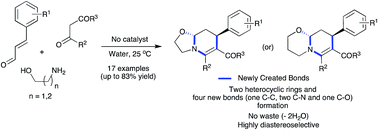
A catalyst-free, three-component reaction between amino alcohols, 1,3-dicarbonyl compounds and α,β-unsaturated aldehydes was developed for the synthesis of fused tetrahydropyridines in water. The β-enaminone formation-initiated domino sequence afforded oxazolo[3,2-a]pyridines and pyrido[2,1-b][1,3]oxazines diastereoselectivity in good yields involving Michael addition, intramolecular cyclization and iminium ion cyclization steps. This environmentally benign protocol is highly atom-economical where the only side product was two molecules of water and no catalyst or reagent was employed. Besides, in a single operation, four new bonds including two C–N, one C–O and one C–C bonds and two heterocyclic rings were created. The reaction was also effective in various green solvents such as glycerol, PEG-200 and lactic acid.

 Please wait while we load your content...
Please wait while we load your content...