Unusual spectroscopic and photophysical properties of meso-tert-butylBODIPY in comparison to related alkylated BODIPY dyes†
Abstract
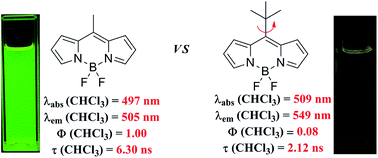
Five alkyl-substituted difluoroboron dipyrrin (BODIPY) dyes have been synthesized: three with a methyl, isopropyl or tert-butyl group at the meso-position of the BODIPY core and two substituted with one or two tert-butyl functions at the 3/5-positions. X-Ray structural analysis, UV-vis absorption spectroscopy and fluorescence (steady-state and time-resolved) techniques have been used to study the structures and the spectroscopic/photophysical properties of these dyes. All but one of these BODIPYs are highly fluorescent in all the solvents tested, the exception being meso-tert-butylBODIPY (2). Derivative 2 differs from the other alkylated boron dipyrrins as it exhibits a broad and red-shifted fluorescence band with a large Stokes shift. In addition, very low fluorescence quantum yields and short fluorescence lifetimes characterize 2. Quantum chemical calculations indicate that 2 has a distorted, nonplanar geometry in the S1 excited state due to the rotation of 8-tert-butyl group. Our results lead us to the conclusion that the torsional rotation about the bond connecting the meso-C and the quarternary C of the tert-butyl group of 2 plays a crucial role in the fast radiationless deactivation of this isomer.


 Please wait while we load your content...
Please wait while we load your content...