Revisited water radiolysis at elevated pH by accounting O3˙− kinetics at low and high LET
Abstract
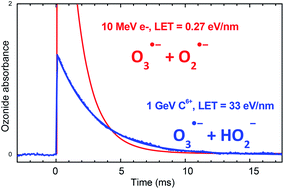
Ozonide radical, O3˙−, is used in this study for probing radiolytic species formed in the radiolysis of liquid water at elevated pH. Its formation allows the scavenging of O˙− whereas its disappearing is due to its reactions with HO2− and O2˙−. This article focuses on the formation and the reaction of O3˙− at pH 13.2 by using pulse radiolysis technique, with 10 MeV electrons and 1 GeV C6+ ions beams for the first time. This allowed us to work with two different Linear Energy Transfer (LET) values (0.27 and 33 eV nm−1 respectively), which means with two different primary distributions of radiolytic species. Consequently the rates constant of reactions O3˙− + HO2− and O3˙− + O2˙− could be revised with acceptable accuracy thanks to deterministic simulations: (1.1 ± 0.2) × 106 M−1 s−1 and (1.5 ± 0.5) × 107 M−1 s−1 respectively. Furthermore, the primary radiolytic yields of HO˙/O˙− and H2O2/HO2− at high LET and pH 13.2 were estimated at 6.5 and 9.6 × 10−8 mol J−1 respectively which corresponds to the literature values.

 Please wait while we load your content...
Please wait while we load your content...