Navigating in chromone chemical space: discovery of novel and distinct A3 adenosine receptor ligands
Abstract
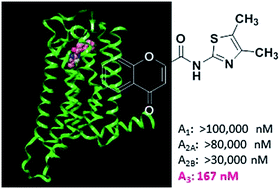
One of the major hurdles in the development of safe and effective drugs targeting G-protein coupled receptors (GPCRs) is finding ligands that are highly selective for a specific receptor subtype. The search for novel compounds with therapeutic value by targeting the A3 adenosine receptor (A3AR) is still in its early stages. The increasing knowledge about the biological, physiological and pathological role of the A3AR subtype was accompanied by the design and development of the A3AR ligands, but the particular role of A3AR agonists and antagonists is still an open issue. Among the large variety of chemical classes screened towards ARs flavonoids have been indicated as remarkable A3AR antagonists. However, the search of A3AR ligands based on this framework seems to be discontinued. In this context, our research group focused its investigation into the discovery and development of novel, potent and selective AR ligands based on the chemical core of flavonoids, the chromone scaffold. The ongoing research has shown that chromone-2-phenylcarboxamide derivatives display a remarkable preference for hA3AR. In this work we report stimulating results, supported by A2A/A3 molecular docking simulations and structure–affinity-relationship (SAR) studies by which N-(4,5-methylthiazol-2-yl)-4-oxo-4H-chromene-2-carboxamide (compound 31) emerged as the most potent and selective compound, displaying an hA3 Ki of 167 nM and a selectivity ratio of 590 vs. the hA1 and 480 vs. the hA2AAR subtypes. The chromone-based ligand was obtained by a simple synthetic approach and will enter in a lead optimization program to enhance its potency and drug-like properties.

 Please wait while we load your content...
Please wait while we load your content...