Arylhydrazones of barbituric acid: synthesis, coordination ability and catalytic activity of their CoII, CoII/III and CuII complexes toward peroxidative oxidation of alkanes†
Abstract
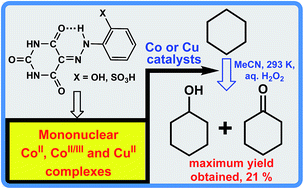
New ortho-substituted arylhydrazones of barbituric acid, 5-(2-(2-hydroxyphenyl)hydrazono) pyrimidine-2,4,6(1H,3H,5H)-trione (H4L1) and the sodium salt of 2-(2-(2,4,6-trioxotetra-hydropyrimidin-5(2H)-ylidene)hydrazinyl)benzenesulfonic acid (H4L2), [Na(H3L2)(μ-H2O)(H2O)2]2 (1), were used in the synthesis of CuII, CoII and CoII/III complexes, [Cu(H2L1)(H2O)(im)]·3H2O (im = imidazole) (2), [Co(H2O)6][Co(H2L1)2]2·8H2O (3), [Co(H2L2)(im)3] (4), [Cu(H2L2)(im)2]·H2O (5) and [Co(H2O)6][H3L2]2·8H2O (6). The complexes are water soluble and the mono- or di-deprotonated ligands display different coordination modes, depending on the synthetic conditions. The electrochemical behaviour of all the compounds was investigated by cyclic voltammetry and controlled potential electrolysis, revealing that the ligands are also redox active. All the compounds were evaluated as catalysts for the peroxidative (with H2O2) oxidation of cyclohexane at room temperature. The compounds 2 and 3 are the most active ones (yields up to 21% and TON up to 213 are achieved, in the presence of 3).


 Please wait while we load your content...
Please wait while we load your content...