Improved electrochemical properties of LiMn2O4 with the Bi and La co-doping for lithium-ion batteries
Abstract
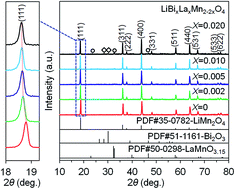
A series of LiBixLaxMn2−2xO4 (x = 0, 0.002, 0.005, 0.010, 0.020) samples were synthesized by solution combustion synthesis in combination with calcination. The phase structure and morphology of the products were characterized by X-ray diffraction, scanning electron microscopy, and transition electron microscopy. The results demonstrated that a single-phase LiMn2O4 spinel structure was obtained for the LiBixLaxMn2−2xO4 (x = 0, 0.002, 0.005) samples, whereas impurities were observed for the LiBixLaxMn2−2xO4 (x = 0.010, 0.020) samples as a result of the doping limit. The electrochemical properties were investigated by galvanostatic charge–discharge cycling and cycling voltammetry in a voltage range of 3.2–4.4 V. The substitution of Mn3+ by equimolar Bi3+ and La3+ could significantly improve the structural stability and suppress the Jahn–Teller distortion, thereby resulting in improved electrochemical properties for the Bi and La co-doped samples in contrast with the pristine LiMn2O4 sample. In particular, the LiBi0.005La0.005Mn1.99O4 sample delivered a high initial discharge capacity of 130.2 mA h g−1 at 1C, and following 80 cycles, the capacity retention was as high as 95.0%. Moreover, it also presented the best rate capability among all the samples, in which a high discharge capacity of 98.3 mA h g−1 was still maintained at a high rate of 7C compared with that of 75.8 mA h g−1 for the pristine LiMn2O4 sample.

 Please wait while we load your content...
Please wait while we load your content...