Synthesis of new porphyrin/4-quinolone conjugates and evaluation of their efficiency in the photoinactivation of Staphylococcus aureus†
Abstract
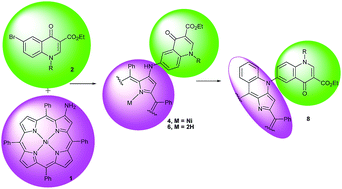
Simple methodologies giving access to a new series of pophyrin/4-quinolone conjugates 6 and to the corresponding intra-cyclized derivatives 8 are described. The key steps to obtain 6 involved palladium-catalyzed amination reactions of 6-bromo-4-quinolones containing N-ethyl, N-pentyl and N-ribofuransyl substituents with (2-amino-5,10,15,20-tetraphenylporphyrinato)nickel(II) followed by demetallation. Compounds 8 were obtained from compounds 4, the nickel(II) complexes of 6, by an oxidative intracylization approach. The new conjugates were fully characterized and the evaluation of singlet oxygen production showed that these compounds possess good to high capability to generate singlet oxygen. The efficacy of these derivatives to photoinactivate Staphylococcus aureus, a Gram-positive bacteria, was evaluated and the best results were obtained with the N-ethyl derivatives 8a and 6a.

 Please wait while we load your content...
Please wait while we load your content...