Diversity oriented synthesis of 6-nitro- and 6-aminoquinolones and their activity as alkaline phosphatase inhibitors†
Abstract
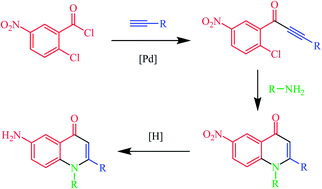
The novel quinolone derivatives synthesized by cyclization of α,β-ynones with primary amines were shown to be promising TNAP and IAP inhibitors. The mechanism of their formation was studied by the isolation of intermediates.

 Please wait while we load your content...
Please wait while we load your content...