Rhodium-catalyzed hydroaminomethylation of cyclopentadiene
Abstract
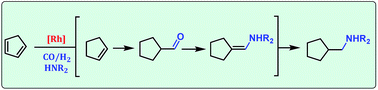
A one-step rhodium catalyzed direct route from cyclopentadiene (Cpd) to saturated C6-amines is presented via hydroaminomethylation (HAM). The homogeneous catalyst of Rh(II) octanoate dimer without additional phosphorus ligands was established giving a 77% yield of the monoamine species of Cpd with pyrrolidine within 4 hours. The unwanted reaction of Cpd, the dimerization to dicyclopentadiene (Dcpd), could be suppressed by careful adjustment of the CO : H2 ratio in the synthesis gas and the choice of solvent, both giving the possibility to steer the reaction to selectively produce amines of either Cpd or Dcpd. Furthermore, other investigated cyclic and non-cyclic aliphatic amine substrates showed excellent selectivity up to 100% for the formation of the HAM-products.

 Please wait while we load your content...
Please wait while we load your content...