Micelle stability in water under a range of pressures and temperatures; do both have a common mechanism?†
Abstract
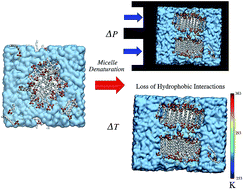
The formation of sodium dodecylsulfate (SDS) micelles in water and heavy water at different pressures and temperatures using molecular dynamics simulations was used to analyze their stability and structure under different conditions and to evaluate the agreement with existing experiments. The results show the assembling of micelles at 1 bar and the presence of larger aggregates under high pressure (over 3 kbar). These large aggregates are not micelles but small finite pieces of bilayers in rod-like shapes. The results obtained using systems at different temperatures showed that both high and low temperatures produce lamellar structures. It is well-known that micelles expose polar residues to water and leave non-polar residues inside where they interact via hydrophobic interactions. High pressure as well as low and high temperatures inhibit the hydrophobic interactions, and under these conditions other structures are produced instead of micelles. This process seems to be similar to protein denaturation under certain temperatures and pressures.

 Please wait while we load your content...
Please wait while we load your content...