Synthesis of N-benzyl-N-phenylthiophene-2-carboxamide analogues as a novel class of enterovirus 71 inhibitors†
Abstract
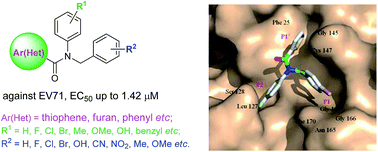
A series of novel human enterovirus 71 inhibitors, N-benzyl-N-phenylthiophene-2-carboxamide analogues, were synthesized and their antiviral activities were evaluated in vitro. Most derivatives of this structure against EV71 had a low micromolar range in the RD (rhabdomyosarcoma) cell lines. The most potent compound 5a, N-(4-bromobenzyl)-N-(4-fluorophenyl)thiophene-2-carboxamide, showed low micromolar activity against EV71 (EC50 = 1.42 μM) compared to the reference anti-EV71 drug enviroxime (EC50 = 0.15 μM). Preliminary SAR studies revealed that the thiophene-2-carboxamide core is crucial for maintaining antiviral activity, and N-substituent phenyl groups largely influenced the anti-EV71 efficacy of this new class of potent antiviral agents.

 Please wait while we load your content...
Please wait while we load your content...