Aqueous phase reforming of ethylene glycol on Pt/CeO2–ZrO2: effects of cerium to zirconium molar ratio†
Abstract
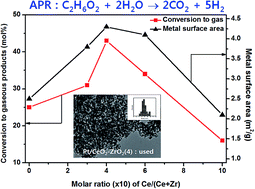
The aqueous-phase reforming (APR) reaction of ethylene glycol was investigated on platinum-supported CeO2–ZrO2 mixed oxides with different molar ratios of Ce/(Ce + Zr). The main role of the molar ratio of Ce/(Ce + Zr) on the CeO2–ZrO2 mixed oxides was found to be to alter the dispersion of active platinum crystallites and concomitantly change the hydrogen productivity and stability. The different amounts of surface oxygen species on the defect sites of CeO2–ZrO2 altered the dispersion of platinum crystallites and changed the metal–support interactions between the nano-scale platinum crystallites and the oxygen vacancy defect sites on the CeO2–ZrO2. A larger dispersion of platinum crystallites and a larger surface area were observed for the Pt/CeO2–ZrO2 having a Ce/(Ce + Zr) molar ratio of 4 : 6, and the catalyst showed a higher conversion of ethylene glycol to hydrogen by forming thermally stable platinum crystallites through their strong interactions with the defect sites of the CeO2–ZrO2 surface.

 Please wait while we load your content...
Please wait while we load your content...