An enantioselective approach to 2-alkyl substituted tetrahydroquinolines: total synthesis of (+)-angustureine†
Abstract
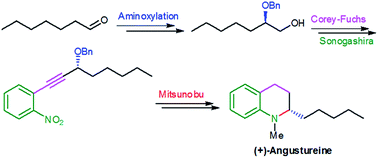
A simple and highly efficient synthetic approach to enantiopure 2-alkyl substituted tetrahydroquinoline 1 skeleton from aldehydes as starting materials and its application to the total synthesis of (+)-angustureine 2 is described. Key transformations include proline catalyzed aminoxylation, Corey–Fuchs protocol, Sonogashira coupling and intramolecular Mitsunobu reactions.

 Please wait while we load your content...
Please wait while we load your content...