Vibrational spectra of solid cis- and trans-2-thioxohexahydroquinazolin-4(1H)-one and theoretical calculations towards the interpretation of its thermal reactivity†
Abstract
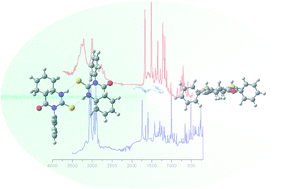
FT-Raman and FT-IR spectra of solid cis- and trans-2-thioxohexahydroquinazolin-4(1H)-one are reported from 4000 to 200 cm−1. The molecular geometry, Wiberg Index, NBO analysis and vibrational wavenumbers in the ground state have been calculated using a density functional method (B3LYP) with 6-31+G** and 6-311+G** basis set. Both compounds are stable as dimers in the solid phase, possessing C2 symmetry. The scaled theoretical wavenumbers showed very good agreement with the experimental values. This work contributes to the knowledge of important data which are rather scarce for quinazolinones.

 Please wait while we load your content...
Please wait while we load your content...