Directly probing the effect of the solvent on a catalyst electronic environment using X-ray photoelectron spectroscopy†
Abstract
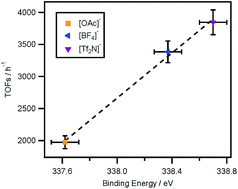
The electronic environment of the metal centre of a catalyst dissolved in ionic liquids has a determining effect on its catalytic efficiency in chemical reactions. However, the electronic environment of the ionic liquid-based metal centres can be influenced by not only their chemical state but also the solute–solvent interaction. In this work, we demonstrate that the anion of an ionic liquid can significantly influence the electronic environment of a metal centre. The metal centre electronic environment can be monitored by measuring the typical electron binding energies by X-ray photoelectron spectroscopy (XPS). The correlation of the electronic environment of the metal centre with reaction performance provides a possibility to design and control a chemical reaction. In this work, we also illustrate a strategy for tuning the electronic environment of metal centres, by the selection of particular ionic liquid anions, to design a catalytic system and consequently to finally control the reaction performance of a model Suzuki cross coupling reaction.

 Please wait while we load your content...
Please wait while we load your content...