Aryl tosylates as non-ionic photoacid generators (PAGs): photochemistry and applications in cationic photopolymerizations†
Abstract
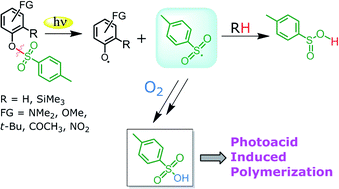
The irradiation of various substituted aryl tosylates was investigated in a solution and homolysis of the ArO–SO2C6H4CH3 bond was the path exclusively observed. The corresponding phenols and photo-Fries adducts were obtained and p-toluenesulfinic and p-toluenesulfonic acids were liberated. The nature and amount of the acid photoreleased were tuned by changing the reaction conditions and the nature and position of the aromatic substituents. In deaerated solutions p-toluenesulfinic acid was formed exclusively, whereas under oxygenated conditions the stronger p-toluenesulfonic acid was released, as shown by HPLC ion chromatography analyses. ArO–S bond photocleavage takes place from the singlet state, as confirmed by laser flash photolysis experiments, and competitive intersystem crossing can make the aryl tosylate unreactive when a nitro group is present. The application of these aryl tosylates as non-ionic photoacid generators (PAGs) in hybrid organic/inorganic sol–gel photoresists has been explored.

 Please wait while we load your content...
Please wait while we load your content...