Synthesis of norbornane bisether antibiotics via silver-mediated alkylation†
Abstract
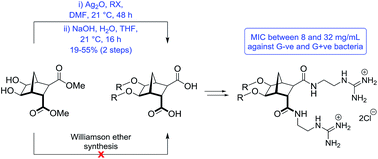
A small series of norbornane bisether diguanidines have been synthesized and evaluated as antibacterial agents. The key transformation—bisalkylation of norbornane diol 6—was not successful using Williamson methodology but has been accomplished using Ag2O mediated alkylation. Further functionalization to incorporate two guanidinium groups gave rise to a series of structurally rigid cationic amphiphiles; several of which (16d, 16g and 16h) exhibited antibiotic activity. For example, compound 16d was active against a broad range of bacteria including Pseudomonas aeruginosa (MIC = 8 μg mL−1), Escherichia coli (MIC = 8 μg mL−1) and methicillin-resistant Staphylococcus aureus (MIC = 8 μg mL−1).

 Please wait while we load your content...
Please wait while we load your content...