Sequential palladium catalyzed coupling–cyclocondensation–coupling (C3) four-component synthesis of intensively blue luminescent biarylsubstituted pyrazoles†
Abstract
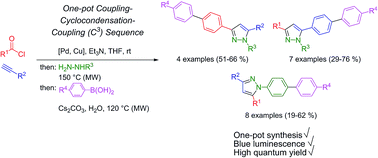
1-, 3-, and 5-Biarylsubstituted pyrazoles can be efficiently prepared by a microwave-assisted consecutive four-component synthesis based upon a sequential Pd-catalyzed coupling–condensation–coupling (C3), a one-pot sequence which concatenates Sonogashira alkynylation and Suzuki arylation intercepted by pyrazole forming cyclocondensation of the ynone intermediate. This diversity-oriented approach enables tailoring, fine-tuning and optimization of absorption and emission properties towards high fluorescence quantum yields in solution up to Φf = 0.97. The increased luminescence intensity stemming from biaryl substitution can be rationalized by ground and excited state computations on the DFT level of theory, which nicely reproduce the experimental data of absorption and emission.

 Please wait while we load your content...
Please wait while we load your content...