Disulfide cross-linked cholic-acid modified PEG–poly(amino acid) block copolymer micelles for controlled drug delivery of doxorubicin†
Abstract
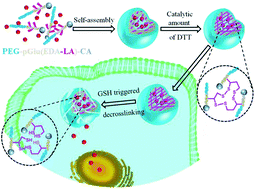
Reduction responsive biodegradable core-cross-linked micelles are developed form lipoic acid (LA) and cholic acid (CA) decorated poly(ethylene glycol)-b-poly(L-glutamic acid) (PEG-pGlu(EDA-LA)-CA) block copolymers and investigated for intracellular doxorubicin (DOX) release. The amphiphilic polymers can self-assemble into nano-sized core–shell micelles that are easily cross-linked in the presence of a catalytic amount of dithiothreitol (DTT). The cross-linked micelles (CLM) show excellent stability against extensive dilution and high salt concentration but rapid dissociation and drug release in reductive environments. Confocal laser scanning microscopy further demonstrates that DOX was delivered and released into the nuclei of HeLa cells following 8 h incubation with DOX-loaded CLM. MTT assays reveal that DOX-loaded CLM had similar anti-tumor activity to non-cross-linked micelles (NCLM) for HeLa cells following 48 h incubation, while blank micelles were practically nontoxic up to a tested concentration of 1.0 mg mL−1. These reduction responsive core-cross-linked micelles have great potential for drug delivery in cancer chemotherapy.

 Please wait while we load your content...
Please wait while we load your content...