Microscopic study of the corrosion behaviour of mild steel in ionic liquids for CO2 capture applications†
Abstract
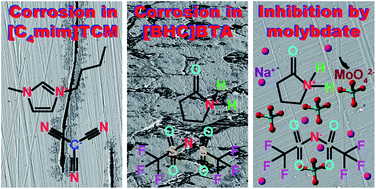
Three 1-alkyl-3-methylimidazolium tricyanomethanide (TCM) ionic liquids (ILs) (alkyl = ethyl, butyl and hexyl) and one butyrolactam cation-based IL with a fluorinated anion were synthesised and tested in contact with mild steel (MS) at temperatures up to 80 °C. The corrosion behaviour was evaluated by monitoring the morphological changes on the steel surface after testing. Exposure of MS to the IL results in two main types of degradation that depend on the IL type. General etching over the macroscopic surface of the alloy was revealed for the IL with the fluorinated anion. The 1-alkyl-3-methylimidazolium TCM ILs promoted dissolution of MnS inclusions present in the steel. In the ILs with a shorter alkyl chain in the cation (alkyl = ethyl, butyl), the dissolution of MnS was accompanied by generation of corrosion products around the inclusion sites, which are mainly identified as magnetite and maghemite ferrites by micro-Raman spectroscopy. The rest of the macroscopic steel surface remains unaffected. Etching resulted in significant weight loss due to removal of material, whereas no significant weight loss was revealed following MnS dissolution. Butyrolactam cation-based IL severely attacks MS with the formation of a plethora of corrosion products including ferrites (mainly hematite), zinc oxide, sulphates and carbonates. Addition of 500 ppm sodium molybdate to the butyrolactam cation-based IL resulted in efficient inhibition of etching at both room temperature and 60 °C due to adsorption of molybdate on the alloy surface. A side effect of MS degradation is that the CO2 absorption capacity of the ILs can be severely reduced through the transfer of metal ions and corrosion products from the metallic surface to the liquid phase. Therefore, gravimetric CO2 absorption capacity and kinetic measurements on the selected 1-alkyl-3-methylimidazolium tricyanomethanide ILs before and after their contact with MS were also conducted with the purpose to unveil and study these side effects. Moreover, CO2 absorption experiments of the butyrolactam cation-based IL before and after contact with MS, as well as in the presence of a sodium molybdate inhibitor, showed that sodium molybdate has the capacity to limit significantly the etching rate without affecting the CO2 capture performance of the IL.

 Please wait while we load your content...
Please wait while we load your content...