Effect of boron–nitrogen bonding on oxygen reduction reaction activity of BN Co-doped activated porous carbons†
Abstract
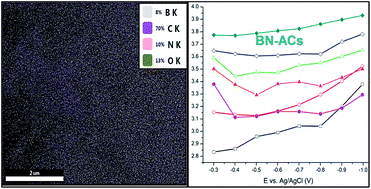
The effect of boron and nitrogen (BN) bonding on the activity of the oxygen reduction reaction was investigated when boron and nitrogen atoms are chemically inserted into activated carbons. With the readily available B and N doping precursors of boric acid and urea, heteroatom-doped carbon materials were synthesized under varying pyrolysis temperatures and mixing ratios of the precursor to activated carbon (AC). According to electrochemical and structural analyses, the heteroatom was doped into the carbon lattice at 1000 °C, whereas annealing temperatures below 850 °C did not achieve the doping. It is striking that the BN co-doped AC shows much higher doping contents than the B or N single-doped AC despite the use of the same amounts of doping precursors as in the single doping case. The electrochemical analysis indicates that higher B or N doping contents enhance the level of ORR activity with the four-electron pathway, especially for the BN co-doping case due to the formation of B–N–C bonds. The results of this study demonstrate the potential of heteroatom-doped AC as a cost-effective alternative catalyst for oxygen reduction reaction in fuel cells.

 Please wait while we load your content...
Please wait while we load your content...