Binding interactions of agents that alter α-synuclein aggregation†
Abstract
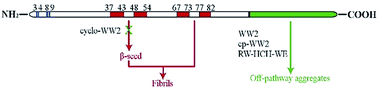
Further examination of peptides with well-folded antiparallel β strands as inhibitors of amyloid formation from α-synuclein has resulted in more potent inhibitors. Several of these had multiple Tyr residues and represent a new lead for inhibitor design by small peptides that do not divert α-synuclein to non-amyloid aggregate formation. The most potent inhibitor obtained in this study is a backbone cyclized version of a previously studied β hairpin, designated as WW2, with a cross-strand Trp/Trp cluster. The cyclization was accomplished by adding a D-Pro-L-Pro turn locus across strand termini. At a 2 : 1 peptide to α-synuclein ratio, cyclo-WW2 displays complete inhibition of β-structure formation. Trp-bearing antiparallel β-sheets held together by a disulphide bond are also potent inhibitors. 15N HSQC spectra of α-synuclein provided new mechanistic details. The time course of 15N HSQC spectral changes observed during β-oligomer formation has revealed which segments of the structure become part of the rigid core of an oligomer at early stages of amyloidogenesis and that the C-terminus remains fully flexible throughout the process. All of the effective peptide inhibitors display binding-associated titration shifts in 15N HSQC spectra of α-synuclein in the C-terminal Q109-E137 segment. Cyclo-WW2, the most potent inhibitor, also displays titration shifts in the G41–T54 span of α-synuclein, an additional binding site. The earliest aggregation event appears to be centered about H50 which is also a binding site for our most potent inhibitor.

 Please wait while we load your content...
Please wait while we load your content...