One-pot synthesis of α-iodoketones from alcohols using ammonium iodide and Oxone® in water†
Abstract
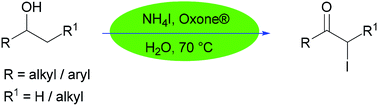
A novel protocol for the synthesis of α-iodoketones from alcohols has been developed. Using water as the reaction medium, ammonium iodide and Oxone® was proven to be an efficient reagent system for this reaction and afforded the corresponding α-iodoketones in moderate to good yields. The generality of this reaction was demonstrated with various secondary alcohols such as benzylic alcohols and aliphatic alcohols (acyclic and cyclic).

 Please wait while we load your content...
Please wait while we load your content...