Recyclable BINOL–quinine–squaramide as a highly efficient organocatalyst for α-amination of 1,3-dicarbonyl compounds and α-cyanoacetates†
Abstract
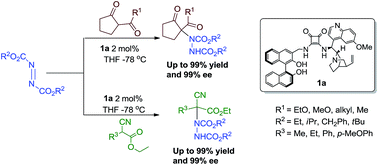
An efficient organocatalytic asymmetric α-amination of 1,3-dicarbonyl and α-cyanoacetates compounds towards chiral α-amino acid precursors is reported. The enantioselective synthesis of these compounds was achieved with excellent yields and ee values (up to 99% yield and 99% ee) by treatment of 1,3-dicarbonyl compounds or α-cyanoacetates with azodicarboxylates in the presence of multiple hydrogen bond donor BINOL–quinine–squaramide organocatalyst 1a developed in our laboratory. The squaramide catalyst 1a can be recovered and reused for four cycles without loss of activity and enantioselectivity.

 Please wait while we load your content...
Please wait while we load your content...