Structural modification by adding Li cations into Mg/Cs-TFSA molten salt facilitating Mg electrodeposition†
Abstract
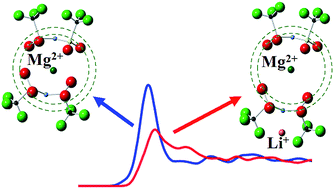
Liquid structures of Li/Mg/Cs-TFSA (TFSA: bis(trifluoromethylsulfonyl)amide, N(SO2CF3)2−) molten-salt systems have been investigated by Raman spectroscopy and high-energy X-ray diffraction. The Raman spectroscopic measurements indicate that the cis-conformer of the TFSA anion is more favorable than the trans-conformer in the molten salts containing Li+ and/or Mg2+, whereas the latter is dominant for a pure Cs[TFSA] molten salt. The present reverse Monte Carlo (RMC) structural modelling suggests that the coordination number of the anion around Mg, NMg-anion, decreased in Li0.1Mg0.1Cs0.8[TFSA]1.1 molten salt compared to that in Mg0.1Cs0.9[TFSA]1.1. The Mg-anion distance under the coexistence of Li+ is found to be about 10–15% longer than that without Li+ at around NMg-anion ∼ 1.5, which means the increase of free volume around Mg cations in the Li-contained molten salt. Thus, the electrodeposition of Mg in alkali-TFSA molten salts would be facilitated under the coexistence of Li cations.

 Please wait while we load your content...
Please wait while we load your content...