Interaction of the core fragments of the LL-37 host defense peptide with actin
Abstract
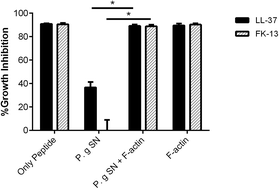
Host defense peptides are effector molecules of the innate immunity that possess antimicrobial and health-promoting properties. Due to their potential therapeutic activities, host defense peptides are being explored as alternatives for antibiotics. The human LL-37 and its shorter, cost-effective, bactericidal core peptide derivates have been suggested for their therapeutic potential. Bacteria evade host defense peptides by proteolytic inactivation. Actin released from necrotized cells and abundant in infected sites was shown to bind and protect LL-37 from microbial proteolytic degradation, and to enable the peptide's antimicrobial action despite the presence of the proteases. Here, we characterized the interactions of the 10–13 residues long LL-37 core peptides with actin. We show that the LL-37 core peptides associate with actin with a lower affinity than that of LL-37. Their association with actin, which is very ionic strength sensitive, is mainly based on electrostatic interactions. Likewise, the antimicrobial activity against Escherichia coli of the minimal antimicrobial peptide KR-12 but not FK-13 nor LL-37 is also very sensitive to salts. In addition, the antimicrobial activity of the FK-13 core peptide is protected by actin against the tested bacterial proteases in a similar manner to that of LL-37, supporting its potential for therapeutic use.

 Please wait while we load your content...
Please wait while we load your content...