UV-vis and EPR spectroelectrochemical investigations of triarylamine functionalized arylene bisimides†
Abstract
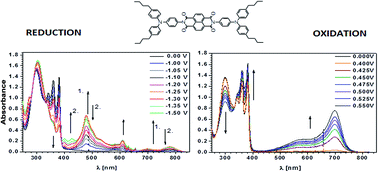
Four arylene bisimides N-substituted with triarylamine and three bisimides core-functionalized with the same substituent were studied by cyclic voltammetry, UV-vis and EPR spectroelectrochemistry. All the investigated compounds showed ambipolar behaviour manifested by their quasi-reversible reduction to radical anions and quasi-reversible oxidation to radical cations. The presence of stable radical anions and radical cations was confirmed by EPR spectroelectrochemical experiments. Formation of the radical anions resulted in bleaching of the bisimide UV-vis bands with simultaneous hypsochromic shift of the charge transfer (CT) band and appearance of the radical anion peaks, the bands originating from the triarylamine remaining essentially unchanged. Electrochemical generation of radical cations resulted in turn in bleaching of the triarylamine band accompanied by a hypsochromic shift of the CT band and with the appearance of the radical cation bands at higher wavelengths, the bisimide bands remained essentially intact.

 Please wait while we load your content...
Please wait while we load your content...