Arsenic removal from aqueous solutions by adsorption using novel MIL-53(Fe) as a highly efficient adsorbent
Abstract
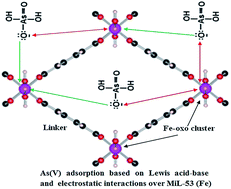
A MIL-53(Fe) analogue was successfully synthesized by a HF free-solvothermal method. The sample was characterized by XRD, N2 adsorption (BET), TEM, FTIR, XPS and AAS. From the N2 adsorption–desorption isotherms, it can be seen that the structure of MIL-53(Fe) in the anhydrous form exhibits closed pores with almost no accessible porosity to nitrogen gas. The XPS results reveal that Fe is really incorporated into the MIL-53(Fe) framework. In the hydrated form, the pores of MIL-53(Fe) are filled with water molecules. Thus, MIL-53(Fe) exhibited a very high adsorption capacity of As(V) in aqueous solution (Qmax of 21.27 mg g−1). Adsorption kinetics data revealed that As(V) adsorption isotherms fit the Langmuir model and obey the pseudo-second-order kinetic equation.

 Please wait while we load your content...
Please wait while we load your content...