Hydrolase-catalyzed asymmetric carbon–carbon bond formation in organic synthesis
Abstract
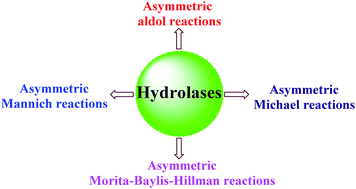
Enzyme catalytic promiscuity, in which the active site of an enzyme has the ability to catalyze more than one chemical transformation, has received widespread attention as more catalytic promiscuities of existing enzymes have been discovered. In this field, hydrolases have been mainly studied due to their commercial availability, high stability, broad substrate scope and high catalytic efficiency in media containing organic solvents. In this study, we review the hydrolase-catalyzed asymmetric carbon–carbon bond-forming reactions for the preparation of enantiomerically enriched compounds in organic synthesis. To date, these hydrolase-catalyzed asymmetric reactions include the direct asymmetric aldol, Michael, Mannich and Morita–Baylis–Hillman reactions. The hydrolase-catalyzed non-enantioselective examples were not included.

 Please wait while we load your content...
Please wait while we load your content...