Reaction of N-heterocyclic carbenes with 13-vertex closo-carboranes: synthesis and structural characterization of zwitterionic salts of 13-vertex nido-carboranes†
Abstract
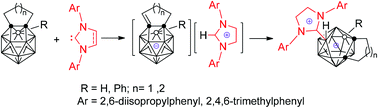
A series of 1 : 1 13-vertex nido carborane–carbene adducts were prepared from the reaction of 13-vertex closo-carboranes with sterically demanding N-heterocyclic carbenes (NHCs) in moderate to very good yields. Single-crystal X-ray analyses reveal that they are zwitterionic salts consisting of the 13-vertex nido-carborane cage and the imidazolium moiety that are connected via a C–B single bond. A mechanistic study shows that 13-vertex closo-carboranes and NHCs undergo a fast acid–base reaction to generate intermediates, i.e imidazolium salts of carborane monoanions, that are slowly converted to the final products. The stability of the intermediates is dependent upon the basicity of NHCs.

 Please wait while we load your content...
Please wait while we load your content...