Intramolecular C–H insertion vs. Friedel–Crafts coupling induced by silyl cation-promoted C–F activation†
Abstract
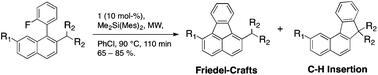
Silyl cation-promoted aryl C–F activation can lead to formal C–H activation and the formation of new C(ar)–C(alkyl) bonds. Deuterium-labeling experiments suggest an insertion of a phenyl cation into the C–H bond. From competition experiments, a relation between reaction rate and C–H bond strength could be established. Mechanistic parallels are drawn to the Mascarelli reaction.
- This article is part of the themed collection: HOT articles in Organic Chemistry Frontiers in 2015

 Please wait while we load your content...
Please wait while we load your content...