Copper-catalyzed stereoselective oxytrifluoromethylation of propargyl amides for the construction of oxazolines†
Abstract
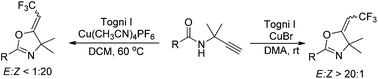
The copper-catalyzed stereoselective oxytrifluoromethylation of terminal propargyl amides took place readily in the presence of Togni's reagent under mild conditions, giving the corresponding trifluoromethylated oxazoline derivatives in moderate to good yields. Using CuBr as catalyst, the E-isomer was obtained as major product. However, the formation of Z-isomer with excellent stereoselectivity was also achieved while Cu(CH3CN)4PF6 was used. Plausible pathways are proposed to account for the excellent stereoselectivity of the reaction.

 Please wait while we load your content...
Please wait while we load your content...