Radical 5-exo cyclization of alkynoates with 2-oxoacetic acids for synthesis of 3-acylcoumarins†
Abstract
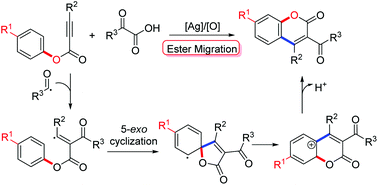
A silver-promoted decarboxylative annulation of alkynoates with 2-oxoacetic acids leading to the formation of 3-acyl-4-arylcoumarins is reported. The process involves radical decarboxylative acylation, 5-exo cyclization, and ester migration.

 Please wait while we load your content...
Please wait while we load your content...