Cation reduction and comproportionation as novel strategies to produce high voltage, halide free, carborane based electrolytes for rechargeable Mg batteries†
Abstract
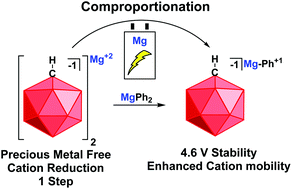
Here we describe the cation reduction and comproportionation as novel routes to synthesize electrolytes for rechargeable Mg-ion batteries. Reduction of the ammonium cation in [HNMe31+][HCB11H111−] with metallic Mg affords the halide free carborane salt [Mg2+][HCB11H111−]2. Comproportionation of [Mg2+][HCB11H111−]2 with MgPh2 affords the novel monocationic electrolyte [MgPh1+][HCB11H111−], which reversibly deposits/strips Mg with a remarkable oxidative stability of 4.6 V vs. Mg0/+2.
- This article is part of the themed collections: Inorganic Chemistry Advancing the Next Generation Batteries and HOT articles in Inorganic Chemistry Frontiers for 2015

 Please wait while we load your content...
Please wait while we load your content...